Write the Rate Laws for the Following Elementary Reactions
Molecularity of elementary steps and corresponding rate laws The molecularity of an elementary step in a reaction mechanism determines the form of its rate law. 2NO 2 g F 2 g 2NO 2 F g NO 2 F 2 NO 2 F F slow F NO 2 NO 2 F fast Explanation.
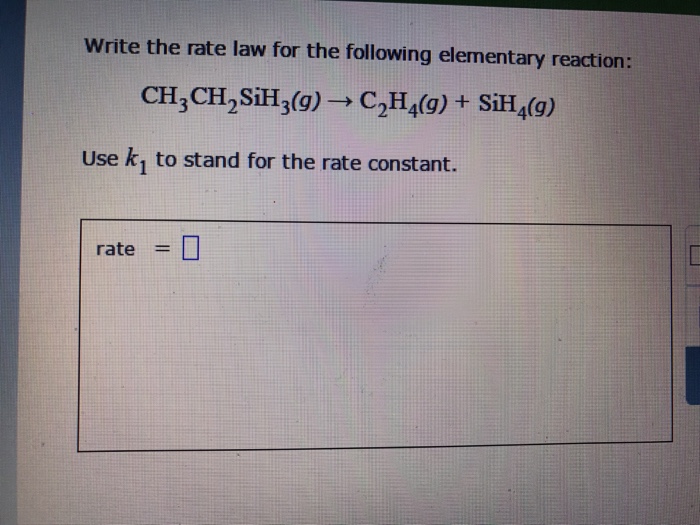
Solved Write The Rate Law For The Following Elementary Chegg Com
Write the rate laws for the following elementary reactions.

. Use k for the rate constant a. Use k for the rate constant a. Rate kCH3NCExplanationIn elementary reactions you use the coefficients of the reactants for the exponents.
Transcribed image text. MathrmCH_3 mathrmNCg rightarrow mathrmCH_3 mathrmCNg b. Write the rate law for the following elementary reaction.
A Write the rate law for the following reactions assuming each reaction follows an elementary rate law. Example of Elementary Steps and Their Rate Laws. Reactants come together to produce the products.
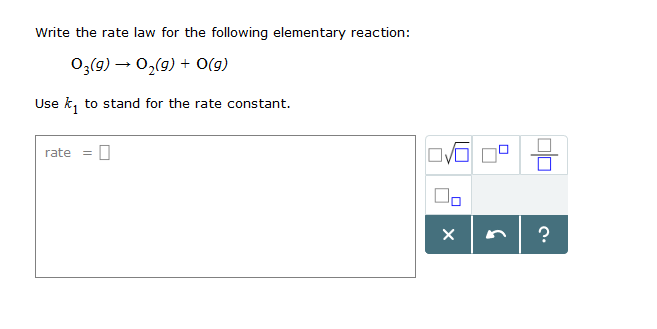
O3 g 02 g Og Rate - d. The rate law is. 136 Write the reaction rate expressions for the following reactions in terms of the disappearance of the reactants and the appearance of products.
Rate fracdCH_3SCH_3dt kNaSCH_3CH_3Br Those two scenarios form the building blocks of rate laws. B Write the rate law for the reaction 2 mathrm Amathrm B rightarrow mathrm C if the reaction 1 is second order in mathrm B and overall third order 2 is zero order in A and first order in B 3 is zero order in both A and B and 4 is first order in. Write the rate law for the following reaction given the reaction mechanism elementary steps.
Other than that its the same. Chemistry questions and answers. O3 g O2 g 0g Ratek d.
O_3g rightarrow O_2gOg d. CH3 NCg CH3 CNg Rate b. Rate law for this is ratekA2.
In the most likely reaction pathway the reaction happens all in one step. O3 g NOg O2 g NO2 g c. For the reaction in the previous example the rate law would be.
Write the rate law by plugging in the reactants into the rate law equation. Use k for the rate constant a. What are the units of the rate constant.
If 2A----B is an elementary reaction. O3g Og 202 g Rate Submit Answer Try Another Version 5 item attempts remaining. Rate law k1 N2O52.
Since step 1 is the slower step it is the rate-determining step for this reaction. O3 g NO g O2 g NO2 g Rate C. Do not include multiplication symbols in the rate equation.
CH3 NCg CH3 CNg Rate b. What are the reaction orders for A and B. Your rate law should not include the SoC1 e 80 Cl 3 states of matter Rate K soc Part 2 1 point See Hint Remember to use square brackets to show concentration.
Write the rate laws for the following elementary reactions. Write the rate laws for the following elementary reactions. 03 g Og 202 g Rate-.
Write the rate laws for the following elementary reactions. What is the overall reaction order. O_3gOg rightarrow 2 O_2g.
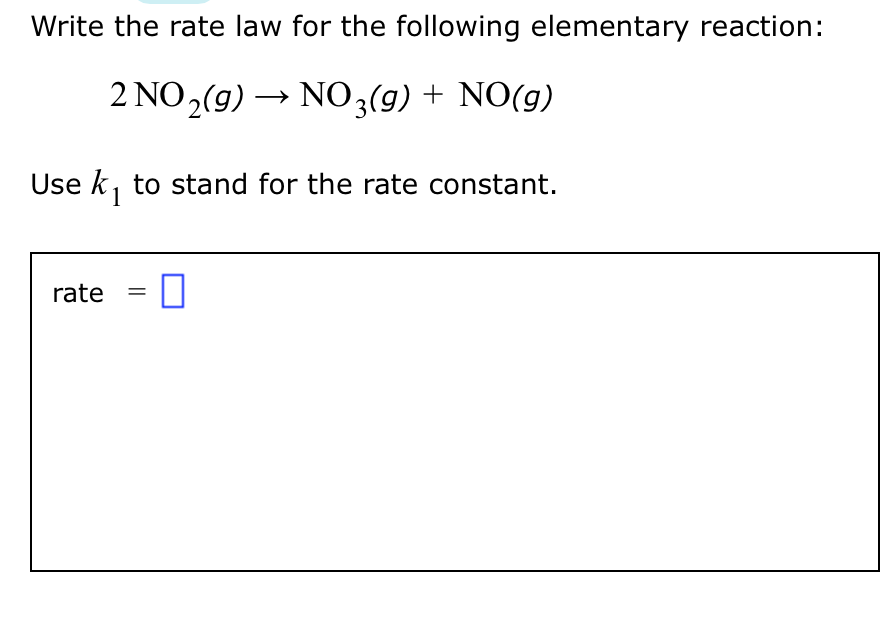
O3 g O2 g O g dO3 g O g 2O2 g 14. A reaction A B C obeys the following rate law. 2 NO 2 g NO3 g NO g Use k1 to stand for the rate constant.
03 g NO g 02 g NO2 g Rate c. HCHH CH-02 C40 CH COOCCHỊ eCH 2CHCOCH nCHiC H0 CHCOCH CHOH CH COCH CHOH 2CHNH CHỊ NH NH CHCO OH0 2CHCOOH b Write the rate law for the following reaction 2AB-C if the reaction is. A proposed mechanism for a reaction is.
Rate kB2 a. O3g O2 g Og Rate d. If A is doubled how will the rate change.
O39 NOg O9 NO2 9 Rate 3D c. A H₂ g I₂ g 2HI g b 5Br aq BrO₃ aq 6H aq3Br₂ aq 3H₂O l The rate expression can be written in terms of any of the reactants or products and should be equivalent. This chemistry video tutorial provides a basic introduction into elementary reactions and elementary rate laws.
CH3 NCCH3 CNg Rate b. Use k for the. 03 g 0g 202 g Rate.
Write the rate laws for the following elementary reactions. A reaction follows an elementary rate law if and only if the iff stoichiometric coefficients are the same as the individual reaction order of each species. Write the rate laws for the following elementary reactions.
If 2NOO 2 2NO 2 then -r NO k NO C NO 2 C O2 if elementary. It explains the difference between unimolecu. C4H9Br C4H9 Br- slow C4H9 H2O C4H9OH2 fast C4H9OH2 H2O C4H9OH H3O fast.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. See the example below for more examples of rate laws. O_3gN Og rightarrow O_2gN O_2g c.
Consider the following reaction. Rate law only cares about reactants and rate law can only be determined experimentally or by using the coefficients of reactants in elementary reactions. An elementary step may involve one molecule or intermediate undergoing a change by itself.
Write the rate law for the following reaction which represents an elementary step in a reaction. Will the rate constant change.

Aleks Writing The Rate Law Of An Elementary Reaction Youtube
Solved Write The Rate Law For The Following Elementary Chegg Com
Solved Write The Rate Law For The Following Elementary Chegg Com
No comments for "Write the Rate Laws for the Following Elementary Reactions"
Post a Comment